ASCO 2024
First Author: Monica Lisi, BS
Abstract Number: 1535
Poster Board Number: 406
Session Information: 6/1/2024, 9:00 AM-12:00 PM CDT
Poster Transcript
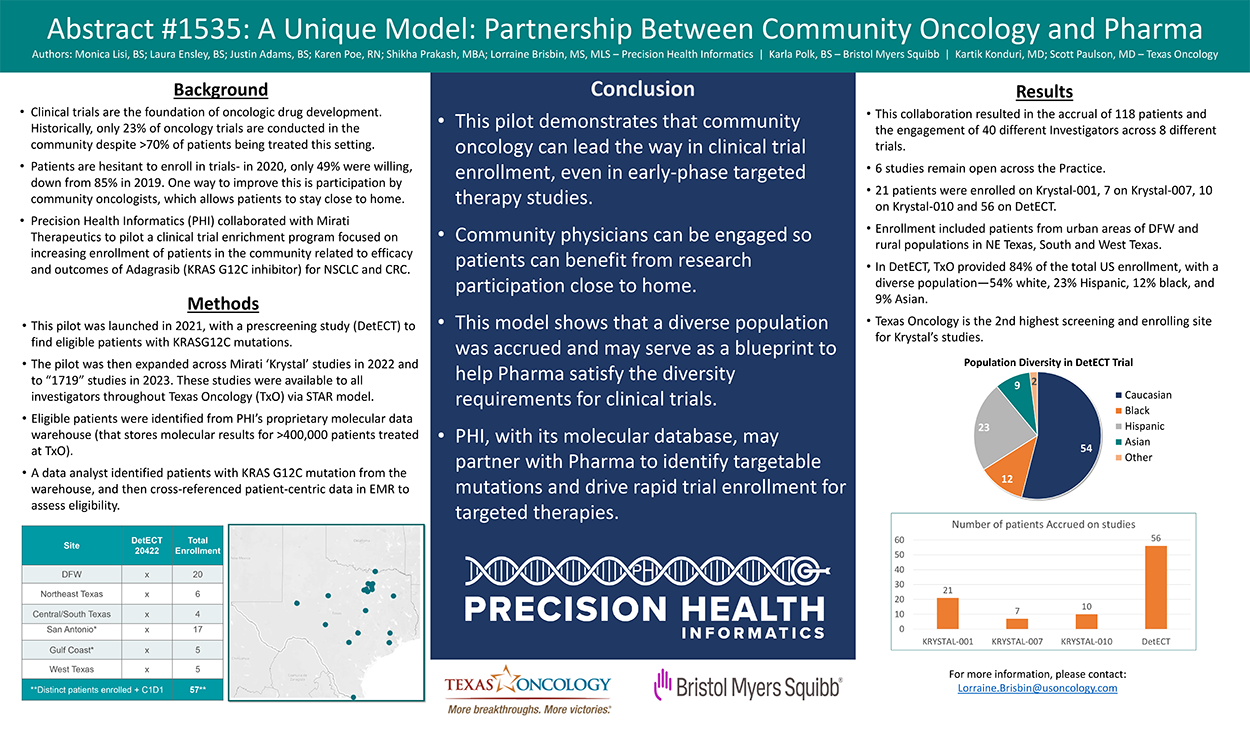
Background: Clinical trials are the foundation of oncologic drug development. Historically, only 23% of oncology trials are conducted in the community despite >70% of patients being treated this setting, leading to a disconnect between the therapy being tested and target population. Also, patients are hesitant to enroll in trials- in 2020, only 49% were willing, down from 85% in 2019. One way to improve this is participation by community oncologists, allowing patients to stay close to home. Targeted therapies are increasingly being approved by the FDA, with 89 small molecule drugs approved by 2020. Given the above landscape, Precision Health Informatics (PHI) collaborated with Mirati Therapeutics to pilot a clinical trial enrichment program focused on increasing enrollment of patients in the community related to efficacy and outcomes of adagrasib (KRAS G12C inhibitor) for NSCLC and CRC.
Methods: This pilot was launched in 2021, with a prescreening study (DetECT) to find eligible patients with KRASG12C mutations. The pilot was then expanded across Mirati ‘Krystal’ studies in 2022 and to “1719” studies in 2023. These studies were available to all investigators throughout Texas Oncology (TxO) via STAR model (clinical trial access to all TxO investigators/sites through a regulated but rapid approval process). Eligible patients were identified from PHI’s proprietary molecular data warehouse (that stores molecular results for >400,000 patients treated at TxO). A data analyst identified patients with KRAS G12C mutation from the warehouse, and then cross-referenced patient-centric data in EMR to assess eligibility. The treating physician was then alerted of their patient’s eligibility. Patient demographic information was also collected from the EMR.
Results: This collaboration resulted in accrual of 118 patients, engagement of 40 different PIs, across 8 different trials. 6 studies remain open across the practice. 21 patients were enrolled on Krystal-001, 7 on Krystal-007, 10 on Krystal-010 and 56 on DetECT. Enrollment was inclusive of patients from urban areas of DFW as well as rural populations in NE Texas, South and West Texas. In DetECT, TxO provided 84% of the total US enrollment, with a diverse population – 54% white, 23% Hispanic, 12% black, 9% Asian. Texas Oncology is the 2nd highest screening and enrolling site on Krystal studies.
Conclusions: This pilot successfully demonstrates that community oncology can lead the way in clinical trial enrollment, even in early phase targeted therapy studies. Community physicians can be engaged so patients can benefit from research participation close to home. This model shows that a diverse population was accrued and may serve as a blueprint to help Pharma satisfy the diversity requirements for clinical trials. PHI, with its molecular database, may partner with Pharma to identify targetable mutations and drive rapid trial enrollment for targeted therapies.